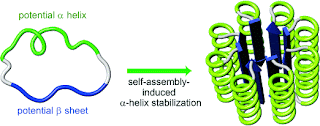
Essentially, the authors combined a peptide with helical tendency and one with beta sheet tendency in a closed loop and found that it forms an alpha/beta structure showing up as roundish particles in the electron microscope. Intriguingly, the open-chain version of this peptide doesn't work, it has to be cyclical.
In natural proteins, this resembles alpha-beta barrel proteins, of which Triosephosphate Isomerase is an example. Note, however, how the sheets and helixes in the natural protein are all twisted, while in the artificial one they are shown as straight and parallel features. If the protein really has this remarkably straight structure, it is most closely related to the architecture of Castel del Monte:

Isn't it amazing how these 13th century architects were ahead of their time ?
I have to point out, however, that the protein structure shown above is based only on the CD spectrum (showing alpha and beta structure) and the EM (showing roundish particles of the correct size for this structure). So far, there seems to be no crystallography or NMR evidence, but I'm hoping the authors will be able to fix that problem soon.
Reference: Y. Lim et al., Ang. Chem. Int. Ed. 48, DOI 10.1002/anie.200804665














No comments:
Post a Comment